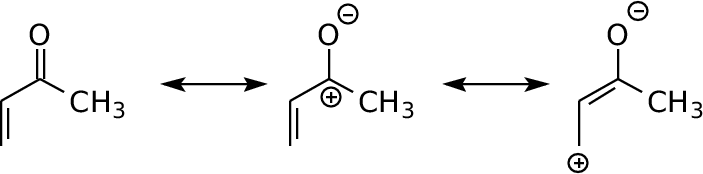
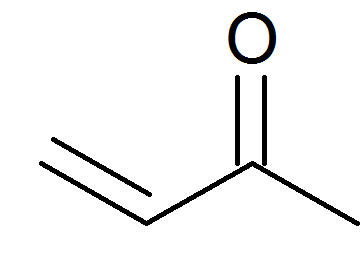
Methyl Vinyl Ketone Resonance Structures
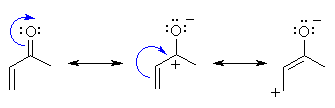
It is found as a volatile in the hawthorn fruit upon ripening and also is part of the scent components of the urine of some animals.
Methyl vinyl ketone resonance structures. Structure ii methyl vinyl ketone mvk binary clathrate hydrates with ch 4 o 2 and n 2 and the effects of secondary guest species on mvk conformation in the cavity of hydrate and on the thermodynamic stability of unirradiated and γ irradiated hydrate phases. Methyl propenyl ketone is occasionally found as a volatile component of normal human biofluids. Fires involving methyl vinyl ketone may be extinguished with dry chemical co2 halon water spray alcohol or standard foam water spray or fog solid streams of water may be ineffective. 5556886 16770722 15739361 914932.
In this work we investigated structure ii methyl vinyl ketone mvk binary clathrate hydrates with ch 4 o 2 and n 2 and the effects of secondary guest species on mvk conformation in the cavity of hydrate and on the thermodynamic stability of unirradiated and γ irradiated hydrate phases. First the rules for resonance structures. The word enamine is derived from the affix en used as the suffix of alkene and the root amine this can be compared with enol which is a functional group containing both alkene en and alcohol ol enamines are considered to be nitrogen analogs of enols. Methyl propenyl ketone is a volatile organic compound.
Draw the curved arrows on the left resonance structure to account for formation of the right resonance structure. The present findings provide meaningful information to. If one of the nitrogen substituents is a hydrogen atom h it is the tautomeric form of an imine. Structure properties spectra suppliers and links for.
The simplest ketone is acetone r r methyl with the formula ch 3 c o ch 3 many ketones are of great importance in industry and in biology. Ae working backwards the last two steps are the formation of a ketone enolate using the strong base lda a k a. Be sure to answer both part 1 and part 2. The required methyl ketone acid 5 hydroxyhexanoic acid is the product of the ozonolysis of alkene c.
Methyl vinyl ketone 78 94 4 ch2 chcoch3. All resonance forms must be or correspond to as in the bond line formalism valid lewis structures. What changes is the formal location of electrons. All nuclear positions are identical in the two forms.
In chemistry a ketone ˈ k iː t oʊ n is a functional group with the structure rc o r where r and r can be a variety of carbon containing substituents ketones contain a carbonyl group a carbon oxygen double bond. Lithium diisopropylamide which is then methylated via an sn2 reaction. Below are two resonance drawings of methylvinyl ketone mvk.